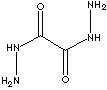
| OXALYL DIHYDRAZIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 996-98-5 |
|
| EINECS NO. | 213-640-2 | |
| FORMULA | H2NNHCOCONHNH2 | |
| MOL WT. | 118.09 | |
| H.S. CODE | ||
|
TOXICITY |
|
|
| SYNONYMS | Oxalic acid dihydrazone; Oxaloyl dihydrazide; | |
| Oxalic acid, dihydrazide; Oxaldihydrazide; Oxalhydrazide; Oxalic acid bishydrazide; Oxalic dihydrazide; Oxalic hydrazide; Oxaloylhydrazine; Oxalyl hydrazide; Oxalylhydrazine; Ethanedioyl dihydrazide; Oxaloylhydrazide; Xalylhydrazide; Ethanedihydrazide; | ||
| DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white to off-white crystalline powder | |
| MELTING POINT |
239 - 243 C (Decomposes) |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER |
|
|
| AUTOIGNITION |
|
|
| VISCOSITY | ||
| PH |
|
|
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 2; Flammability: 0; Reactivity: 0 | |
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Oxalic Acid
(also called Ethanedioic Acid) is a colourless, crystalline, toxic organic
compound belonging to the family of dicarboxylic acids; melting at 187 C;
soluble in water, alcohol, and ether. It occurs in the form of its metal salts
(usually calcium or potassium) in many plants. It is commercially manufactured
by heating sodium formate in the presence of an alkali catalyst to form sodium
oxalate, which should be converted to free oxalic acid when treated with
sulfuric acid. It is also prepared by oxidizing carbohydrates with nitric acid,
by heating saw dust with caustic alkalies or by fermentation of sugar solutions
in the presence of certain molds. Oxalic acid is the only possible compound in
which two carboxyl groups are joined directly; for this reason oxalic acid is
one of the strongest acids in organic compounds. Unlike other carboxylic acids,
oxalic acid (and formic acid) is readily oxidized and combine with calcium,
iron, sodium, magnesium, or potassium to form less soluble salts called
oxalates. Oxalic acid and oxalates are useful as reducing agents for
photography, bleaching, and rust removal. They are widely used as an purifying
agent in pharmaceutical industry, precipitating agent in rare-earth metal
processing, bleaching agent in textile and wood industry, rust-remover for metal
treatment, grinding agent, waste water treatment. acid rinse in laundries and
removing scale from automobile radiators.
Hydrazine (anhydrous) is a clear, fuming, corrosive liquid with an ammonia-like odor; melting at 1.4 C, boiling at 113.5 C, specific gravity 1.011. It is very soluble in water and soluble in alcohol. It decomposes on heating or exposure to UV to form ammonia, hydrogen, and nitrogen, which may be explosive with a blue flame when catalysed by metal oxides and some metals such as platinum or Raney nickel. It is prepared from ammonia with chloramine in the presence of glue or gelatin (to inhibit decomposition of the hydrazine by unreacted oxidants) as the hydrate form usually (100% monohydrate contains 64% by weight hydrazine). Hydrazine is also prepared from sodium hypochlorite with urea in the presence of glue or gelatin. Botht ammonia and amines are nitrogen nucleophiles which donate electrons (they are Lewis bases). But hydrazine (diamine) has much stronger nucleophilicity which makes it more reactive than ammonia. Hydrazine has dibasic and very reactive properties. Hydrazine is used as a component in jet fuels because it produce a large amount of heat when burned. Hydrazine is used as rocket fuel. Hydrazine is used as an oxygen scavenger for water boiler feed and heating systems to prevent corrosion damage. Hydrazine is used as a reducing agent for the recovery of precious metals. It is used as a polymerization catalyst and a chain extender in urethane coatings. It and its derivatives are versatile intermediates. They have active applications in organic synthesis for agrochemicals, pharmaceuticals, photographic, heat stabilizers, polymerization catalysts, flame-retardants, blowing agents for plastics, explosives, and dyes. Recently, hydrazine is applied to LCD (liquid crystal displays) as the fuel to make faster thin-film transistors. Hydrazide is an acyl hydrazine. Acyl (-CO) is an organic radical formed by removal of a hydroxyl group from an organic acid (carboxyl group). |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to off-white crystalline powder | |
| ASSAY |
98.0% min |
|
|
NITROGEN CONTENT |
46.5% min |
|
| MELTING POINT |
239 - 243 C |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
Hazard Symbols: , Risk Phrases: , Safety Phrases: 24/25 |
||